Our comprehensive “Execution Guide Library” contains a wealth of useful guides not available anywhere else on the internet. Prime examples include The Ultimate Guide to Insulin Dosing, Exercise & Diabetes Management, Flexible Dieting with Diabetes, The Diabetics Guide To Bodybuilding and Strength, How to Become a CGM PRO and much more. These guides will answer many of your biggest unanswered questions, and provide unique insight into important elements you have not even considered.
You're Invited...
Join 1000's of diabetic members today and get cutting edge knowledge, workouts, diets, recipes, webinars and more
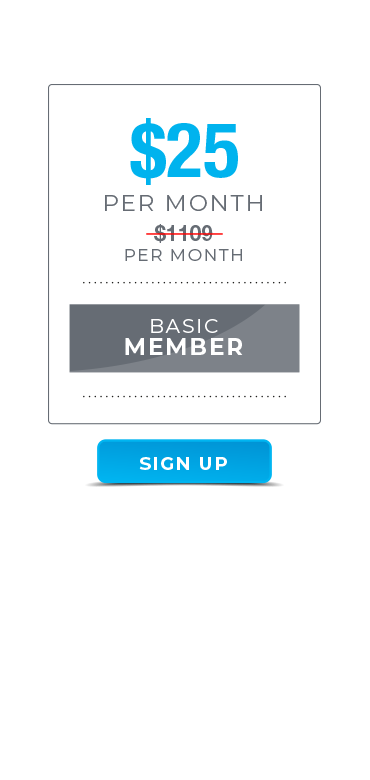
A cutting-edge members’ site for dedicated MEN and WOMEN living with Diabetes who want to build a healthier, stronger better looking body. Join for only $25 p/month – no strings attached, cancel anytime!
Who Is The Training Lab For?

Avid Gym Goers, and anyone into fitness and healthy eating. From Beginner to Pro - all experiences accounted for.










Strength and Physique athletes living with diabetes..Competitive and recreational - it doesn't matter. If you lift weights you joining this community is a must.










Health professionals and coaches who want to learn more about building muscle and shredding fat with diabetes.










Nerdy Fitness enthusiasts who want to keep up to date with the latest evidence based research,
Here's What You Get

EASY TO FOLLOW
EXECUTION GUIDES
IN-DEPTH ARTICLES, VIDEOS AND
RESEARCH REVIEWS
The articles and research reviews section cover the latest studies and media that are directly relevant to you. They are summarised concisely in a format that speaks to YOU, at YOUR level. The videos will mostly deal with important concepts of personal development, managing your diabetes, nutrition, strength training, and body composition.



NETWORK AND CONNECT
Ask questions, network and exchange ideas with thousands of other like minded individuals from every country around the globe, you’re sure to find someone who “gets you.”
EVER GROWING KNOWLEDGE AND EXPERIENCE AT YOUR FINGER TIPS
Not only is the content found in the Diabetic Muscle and Fitness Training Lab exclusive and not available anywhere else. You’ll be receiving the most up to date information on health, motivation, muscle building and fat loss. Not only that, it will be delivered in a way that is applicable and actionable for people with Diabetes.




THE BEST MINDS IN THE FITNESS INDUSTRY
Not only will the resident advice teach you a thing or two. The Training Lab will be interviewing leading experts in the field of diabetes, training, nutrition and psychology, to talk shop on important topics you need to know about.
EMPOWERMENT
Learning is a participation sport. You must take action on what you learn within the Training Lab. All the Execution Guides have pre-prepared study guides and examinations to pass before moving on. To make yourself accountable, you can compare test scores with the community, if you are a competitor!


Why Should You Care About This Site?
Finding credible advice on eating and training to get in shape with diabetes is remarkably hard to find, even on the internet. Your doctor isn't that supportive of your goals and doesn't know or care much about getting in shape,. Also, others without diabetes won't understand your daily challenges. It's also nice to know you aren't living alone with this crazy condition and that other people know exactly how you feel.
Why It's Worth Paying For?










This site will save you a lot of time, money and effort. All the academic learning, thousands of gym hours and commitment and plenty of mistakes have been done before you, documented and presented in an easy to digest fashion for your own benefit.










Cuts through the growing noise and gets to the point.










We're confident that the DMF Training Lab has more informative and practical value than the vast majority of workshops or seminars out there, and overly complicated clinical texts that only a very small proportion of the population could understand.










Finally, all of us at Diabetic Muscle and Fitness are highly educated on sports and clinical nutrition, exercise physiology whilst also being dedicated diabetic athletes and coaches with at least a decade of coaching and learning experience.










That combo is pretty dam rare in the fitness industry. Our combination of both theoretical and practical expertise is what helps set the Diabetic Muscle and FItness Training Lab apart.
A lot of people who post about exercise, diet and supplements for diabetes don’t have a clue what they are talking about, bar the price of the special detox drink they are selling.
It’s hard to put a price on learning new, useful information and discarding incorrect information.
However, the Training Lab holds its on as it focuses exclusively on the goals of controlling diabetes, building muscle and reducing fat.


JOIN THE DIABETIC MUSCLE AND FITNESS
TRAINING LAB TODAY!
Get access to EVERYTHING you need to about building a better body with diabetes and building a better you all round. What are you waiting for?
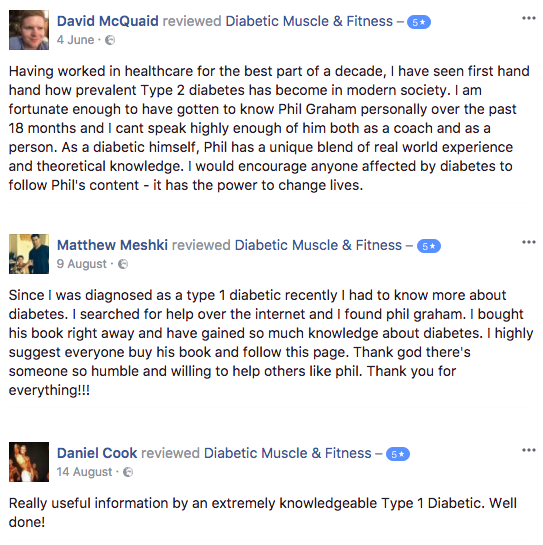
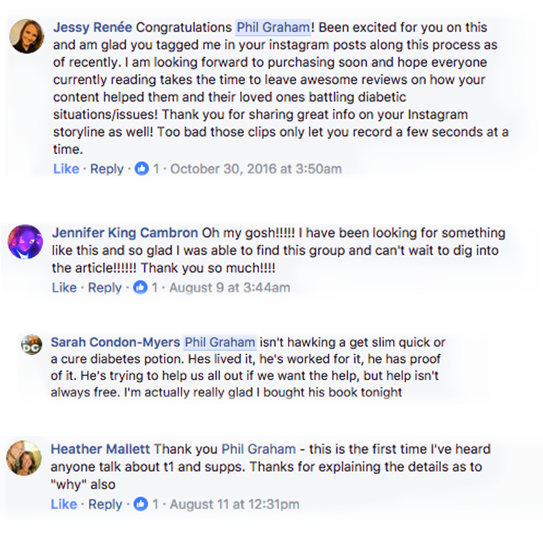
SEE WHAT OUR TRAINING LAB MEMBERS THINK!
*Individual results may vary.
*Individual results may vary.
*Individual results may vary.
*Individual results may vary.
*Individual results may vary.
*Individual results may vary.
*Individual results may vary.
*Individual results may vary.


JOIN AS A FULL MEMBER AND GET THESE SPECIAL GIFTS


Gift #1
The Iconic Insulin & Kilos shirt (Ladies version also)
The iconic Insulin & Kilos T-Shirt is the official and fastest selling t-shirt of Diabetic Muscle and Fitness.
With its clear statement design and a soft blend of comfortable fabrics for a well-formed fit. Represent and let people know who you are in style. …Upgrade your trial today and I’ll rush you an “Insulin & Kilos” t-shirt. The ladies version is in V neck stlye!
That’s not all! I’ll also include…


Gift #2
Diabetic Muscle & Fitness Protein Shaker
Hydrate and Replenish your body with a super smart and slick Diabetic Muscle and Fitness Sports Supplement and Training Shaker.
Use it for Water, Whey, Casein and BCAA’s Pre-Post Workout Cocktails.
Choose your upgrade option below and I’ll rush your t-shirt and shaker to you.


Gift #3
Two Months FREE Membership to the Diabetic Muscle and Fitness Training Lab
You read right. Become a full member today and I will personally grant you two months membership absolutely free.
AS SEEN ON
























-
Access to Highly Comprehensive Video Execution Guides on Diabetes, Medication, Nutrition, Training and Mindset (Worth $299+)
-
Articles covering the biggest unanswered questions about managing diabetes (Worth $99)
-
Downloadable diet, training and diabetes management tools (Worth $99)
-
A physical welcome pack sent direct to your front door (Worth $49)
-
Access to Phil every day of the week inside the member’s community (Worth $99)
-
New workout plans every single month (Worth $99)
-
New delicious, diabetic friendly, high protein recipes every month for members (Worth $49)
-
Live Members Q&A Webinars each month (Worth $99)
-
Access to world expert Interviews in the fields of diabetes, nutrition, mindset and exercise (Worth $199)
-
Early bird access and discount for Online Coaching and Workshops (Worth $99)
Total Value = $1109 per month of value for just $25 per month
Once a member of the Diabetic Training Lab you will get to know the exact diet, training and lifestyle secrets you've been dying to know since your earlier days when first diagnosed.
FREQUENTLY ASKED QUESTIONS
Instant. The moment you sign up to the Diabetic Muscle and Fitness Training Lab + you will be emailed your personal login details to access all the content inside the members site.
As soon as you join the Diabetic Muscle and Fitness Training Lab you get access to 100s of hours of nutrition, training plans, personal development and diabetes focused content.
At Diabetic Muscle and Fitness Training Lab we are mindful of ‘information overload’ and overwhelming our members.
New content is produced and uploaded to the Training Lab every 7-14 days. This gives you appropriate time to study, digest and apply in between.
Both the monthly and annual memberships are recurring payments and can be cancelled at any time just like Netflix, amazon prime or another subscription site.
The annual membership is recurring every 365 days from the first payment.
The monthly membership is recurring ever 30 days from the first payment.
Your Free Diabetic Muscle and Fitness Insulin and Kilos t-shirt and protein shaker will be shipped immediately after you sign up for the annual full time membership option.
Your Training Lab goodies are shipped from Ireland. Delivery times may vary from 1 day to 3 weeks depending on where you live in the world.
The Diabetic Muscle and fitness Training Lab is suitable for men and women of all ages, shapes and ethnicities who want to lose body fat, gain muscle mass, increase strength and become more self-aware of managing their diabetes.
All the diet and training information found inside the Diabetic Muscle and Fitness Training Lab is supported by the most relevant scientific evidence where possible.
I have spent a significant amount of time analysing all the current diabetic research on health, fitness and strength training. None of the studies I’ve used to back up my claims have been cherry-picked.
Prescribing the best diet and training approach is never a matter of a ‘yes’ or ‘no’ answer. There is always a degree of certainty or doubt. For areas that lack research, we can only hypothesize based on what we know, using the closest available research and – where appropriate – trial and error. This is where my personal experience comes in.
Another hugely beneficial aspect of the Diabetic Muscle and Fitness Training Lab is the fact it has been created by someone living with diabetes for people with diabetes.
I directly relate to the underlying frustration and ever-changing challenges people with diabetes face. Not many diabetes fitness or nutrition websites offer that.
Credible advice on diabetes isn’t easy to find. The information and advice you’ll read here will allow you to make more efficient and effective use of your time, money and effort. I know only too well how easy it is to waste these precious resources on poor information.
The effort and detail I go into here should leave you with plenty of time, spare change and energy to direct on other areas of your life. Life is for living!
When it comes to helping people with diabetes reach their full potential.
I don’t mess around.
Look at the books, guides, social media content, podcasts and videos I have produced already. Every one of these is lined with valuable knowledge and experience that can change your life.
If you have read the book you already know the level of detail I go to.
You are about to learn x10 more inside the Diabetic Muscle and Fitness Training Lab and get to connect with many like-minded people.
1000s of people can’t be wrong.
Yours in love, health and strength.
Phil Graham